From Culture to Code: Reinventing Microbial Testing with Bioeureka ™
Introduction
Foodborne illnesses continue to pose a serious global health and economic challenge, affecting nearly 600 million people each year and costing the food industry billions of dollars in recalls, legal liabilities, and loss of consumer trust (WHO, 2023). In today’s high-stakes environment, the speed and accuracy of pathogen detection are critical. Yet, conventional methods remain slow, labor-intensive, and centralized, delaying vital decisions around food safety, compliance, and product release.
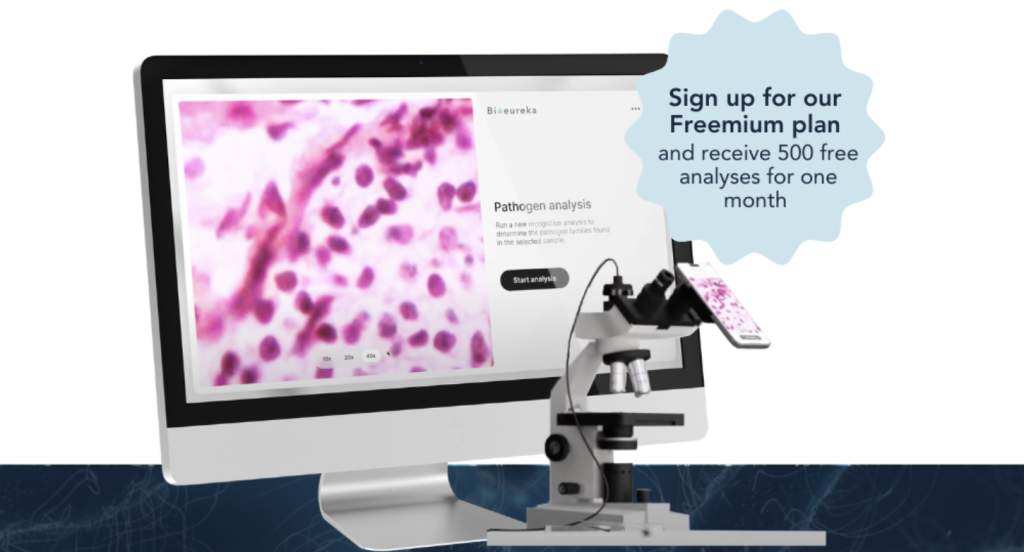
Bioeureka™ introduces a new paradigm. Our AI-powered diagnostic platform enables near-instantaneous detection of foodborne pathogens using high-resolution microscope images – delivered through an intuitive cloud-based interface. Designed to complement and accelerate existing laboratory workflows, Bioeureka™ dramatically reduces turnaround times, operating costs, and reagent use, while maintaining the high accuracy required by the food and health industries.
The Challenge: Outdated Detection Processes
Current microbial detection methods are effective but constrained by manual steps. Traditional workflows depend on culturing pathogens on selective agar plates, followed by repeated chemical tests, and expert interpretation. These steps typically require 48 to 120 hours and must be conducted by trained personnel in centralized facilities. This delay not only slows down compliance efforts but increases the risk of contaminated products reaching the public. In an era when rapid traceability and food chain transparency are essential, this lag poses a significant operational risk.
The Bioeureka™ Solution
Bioeureka’s AI-based platform addresses this challenge head-on by automating the identification and interpretation of bacterial growth from microscope images. The system uses powerful image-based AI models trained on thousands of annotated samples across key microbial species commonly encountered in food processing environments. Users can simply upload images captured with a standard lab microscope or imaging device. The AI model, combined with a logical decision tree that accounts for the type of agar used, interprets the results in real time and returns actionable insights within minutes.
Each customer is provided with a dedicated cloud workspace that enables secure upload, analysis, and tracking of microbiological data. Upon activation, new users receive 500 complimentary test credits, allowing them to explore the system without barriers and evaluate its potential alongside their existing workflows. Whether accessed on-site or remotely, Bioeureka™ empowers lab teams to process samples faster and with fewer consumables.
Use Cases and Value in the Field
Bioeureka’s solution is ideally suited for food safety labs, quality assurance teams, and contract testing facilities seeking faster compliance and better decision-making. It can be used for verifying batch-level safety prior to release, increasing throughput, and reducing the duration of product holds. Educational institutions and training centers also benefit from the platform’s ability to provide immediate feedback on microbial growth, which enhances learning and reduces waste. In healthcare settings, rapid detection and classification can support timely infection control measures without the delays associated with third-party labs.
Environmental Sustainability
Beyond its operational benefits, Bioeureka™ offers significant advantages in terms of sustainability. Traditional microbial testing methods rely heavily on plastic disposables and chemical reagents, which contribute to lab waste and hazardous material disposal. Bioeureka’s image-based workflow reduces the use of single-use plastics and chemical reagents by up to 60%, aligning with the environmental, social, and governance (ESG) goals of modern food and health organizations. In addition, by enabling rapid on-site testing, the platform reduces the need to ship samples to external labs, further cutting transportation-related emissions.
Performance and Competitive Edge
Bioeureka’s performance has been independently benchmarked in collaboration with leading Canadian laboratories. In comparative testing, the platform reduced diagnostic turnaround time by over 85% and achieved over 92% accuracy in correctly identifying microbial strains using microscope imagery alone. Unlike open-source or academic AI tools, Bioeureka™ offers a commercial-grade, validated solution built specifically for food safety and microbiological workflows.
Many alternative solutions fall short in key areas – they lack curated datasets, regulatory validation, commercial integration, or support for onboarding and compliance. Bioeureka™ was purpose-built to address these gaps with a scalable, robust, and user-friendly system that integrates directly into existing lab environments.
Learn More
Bioeureka™ is already helping labs and processors reimagine pathogen detection with speed, precision, and intelligence. Whether you’re seeking operational efficiency, ESG impact, or simply better results, our platform is ready to deliver.
To learn more or request a live demo, visit us at https://bioeureka.com/.
References
- World Health Organization. (2023). Food safety fact sheet.
- Centers for Disease Control and Prevention (CDC). (2023). Foodborne illnesses and outbreaks.
- Dobbyn, T. (2023). How artificial intelligence may improve food safety. UC Davis College of Engineering News.
- Baranyi, J., Rockaya, M., & Ellouze, M. (2024). From data to models and predictions in food microbiology. Current Opinion in Food Science 2024, 57:101177.
- Zhang, H., Wisuthiphaet, N., Cui, H., Nitin, N., Liu, X., & Zhao, Q. (2021). Spectroscopy approaches for food safety applications: Improving data efficiency using active learning and semi-supervised learning. Front. Artif. Intell., 5:863261
- Tille, P. 2014. Bailey and Scott’s Diagnostic Microbiology (13th edition).
- Gerhardt, P., R.G.E. Murray, W.A. Wood and N.R. Krieg. Methods for General and Molecular Bacteriology. ASM Press.
- MacFaddin, J.F. 1985. Media for Isolation-Cultivation-Identification-Maintenance of Medical Bacteria. Vol. 1.
- European Pharmacopoeia 6.5 (2009). 2.6.13 Microbiological examination of non-sterile products: Test for specified
- microorganisms.
- United States Pharmacopoeia 32 NF 27 (2009). <62> Microbiological examination of non-sterile products: Test for specified microorganisms.
- Japanese Pharmacopoeia 4.05 (2008). Microbiological examination of non-sterile products: Test for specified microorganisms.
CLICK HERE TO DOWNLOAD THE WHITE PAPER!
Contact us today:
Devien Durbano,
Microbiologist, Lead Scientist, Bioeureka
Canada & USA: +1-613-614-6323
©2025 Technologie Bioeureka Inc. All rights reserved.