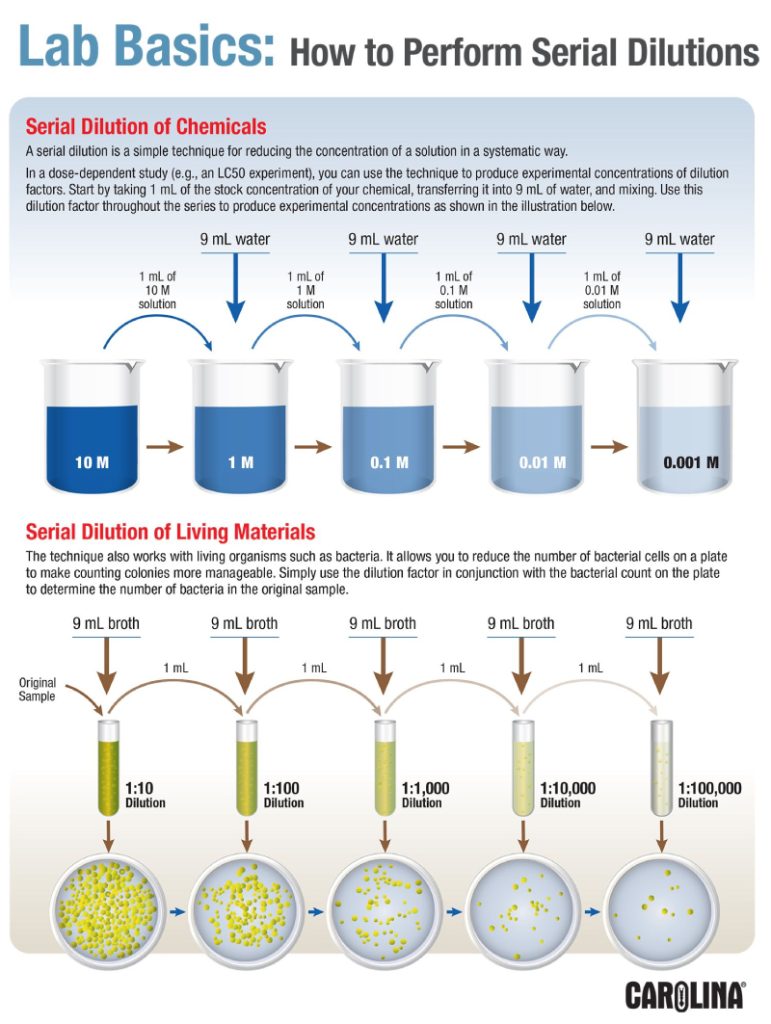
Serial dilution is one of the foundational techniques in microbiology, biochemistry, and analytical chemistry. This simple yet powerful method allows scientists to create a range of solutions with decreasing concentrations, essential for experiments that require precision and control. Whether you’re determining bacterial counts, testing enzymatic activity, or studying toxicology, serial dilution is indispensable.
🔑 Key Steps in Serial Dilution
1️. Prepare the Diluent
Start by selecting an appropriate diluent, such as sterile water, buffer, or culture medium, depending on your specific application. The diluent ensures that the solution’s concentration decreases systematically with each step.
2️. Set the Dilution Factor
Determine the dilution factor based on your experimental needs. Common ratios include:
- 1:10: Each solution is 10 times more dilute than the previous one.
- 1:2: Each solution has half the concentration of the previous step.
3️. Perform the Dilution
- Add a fixed volume of the original solution (e.g., 1 mL) to a fixed volume of the diluent (e.g., 9 mL for a 1:10 dilution).
- Mix thoroughly to ensure uniformity.
- Transfer a fixed volume from the diluted solution to the next tube containing fresh diluent, repeating the process across a series of tubes.
4️. Label the Dilutions
Clearly label each tube with its dilution factor or concentration. Proper labeling avoids confusion and ensures reproducibility.
🔬 Example: A Serial Dilution Protocol
Starting Solution: 1 M
Dilution Factor: 1:10
Number of Dilutions: 5
- Tube 1: Add 1 mL of the original solution to 9 mL of diluent → 0.1 M.
- Tube 2: Add 1 mL from Tube 1 to 9 mL of diluent → 0.01 M.
- Tube 3: Add 1 mL from Tube 2 to 9 mL of diluent → 0.001 M.
- Tube 4: Add 1 mL from Tube 3 to 9 mL of diluent → 0.0001 M.
- Tube 5: Add 1 mL from Tube 4 to 9 mL of diluent → 0.00001 M.
Each step reduces concentration systematically, providing a range of solutions for testing.
🤔 Applications of Serial Dilution
- Microbiology
Serial dilution is used to prepare bacterial suspensions for colony counting. By plating dilutions onto growth media, scientists can estimate the number of bacteria in the original sample. - Biochemistry
This technique is essential for diluting enzymes, substrates, or reagents to precise concentrations needed for biochemical assays. - Toxicology
Serial dilutions help determine the concentration at which a toxic substance produces its effect, known as the dose-response relationship.
🌟 Why Serial Dilution Matters
Serial dilution is more than a routine lab task—it’s a gateway to precision and discovery. By breaking down complex solutions into manageable concentrations, it enables researchers to quantify, test, and explore with accuracy. Whether you’re studying bacterial populations or testing chemical reactions, mastering serial dilution is a skill that drives scientific progress.
For every lab professional or aspiring scientist, this technique is an invaluable tool to have in your arsenal. As a biochemist, microbiologist, and immunologic, I have seen firsthand how this method underpins countless experiments, turning abstract ideas into measurable outcomes.
Let’s continue pushing the boundaries of discovery, one dilution at a time.
– Mohamed M. Diallo
Biochimiste, Microbiologiste, Immunologue